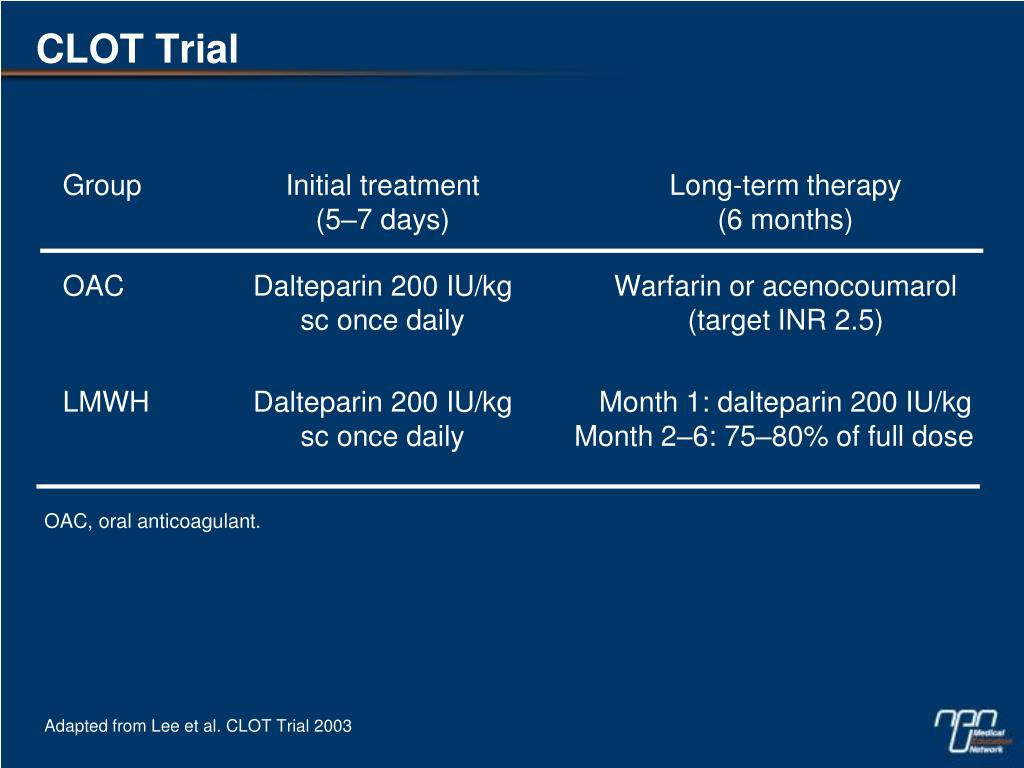
P Clot Trial
The ATTRACT Study was a large clinical trial that compared two commonly-used DVT treatment strategies (blood-thinning medications alone or blood-thinning medication plus catheter-directed clot removal) to determine which is best. This landmark multi-disciplinary initiative was strongly endorsed by the Office of the U.S. Surgeon General because of the study goal to improve patient outcomes, a key research priority noted in the Surgeon General’s Call to Action on DVT.


Why Was the ATTRACT Trial Performed?
The ATTRACT Trial addressed a major controversy among doctors regarding the best way to treat patients with proximal deep vein thrombosis (DVT, blood clots of the upper leg and pelvis). It was known that even when standard blood-thinning drugs are used, about 40% of patients will develop post-thrombotic syndrome (PTS). Because PTS results from permanent damage to the leg veins that is caused by the blood clots, some doctors have urged their immediate removal by a procedure that delivers clot-busting drugs and devices, in addition to blood thinning-drugs.
There was a non-significant reduction in the recurrence of VTE in the edoxaban group (7.9% vs. 11.3%; P = 0.09), coupled with a higher rate of major bleeding (6.9% vs. 4.0%; P = 0.04). Bleeding was primarily seen in patients who entered the trial with gastrointestinal cancers. Tissue plasminogen activator (abbreviated tPA or PLAT) is a protein involved in the breakdown of blood clots. It is a serine protease (EC 3.4.21.68) found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Original Article Jan 13, 2021 Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine J. Sadoff and Others More from the week of December 23, 2010. The CLOT trial established dalteparin as being a superior anticoagulant over vitamin K antagonist therapy and it was the pivotal study that led to the regulatory approval of dalteparin for the extended treatment of thrombosis in cancer patients.
On the other hand, the clot-busting drugs can cause bleeding, and there were no well-designed, large studies showing that these new treatments truly improved patient health in the long run.
So, the main reason why the ATTRACT Trial was performed was to determine if eliminating blood clots with these procedures was effective in preventing PTS, with acceptable safety and costs. Because PTS is a common problem that affects many thousands of patients each year, the ATTRACT Trial was hailed as a landmark study, and its conduct was strongly endorsed by multiple health professional organizations and the Office of the United States Surgeon General.
What Is the Current Status of the Study?
The ATTRACT Trial successfully enrolled its full target of 692 patients and closed to enrollment on December 16, 2014. The 2-year patient follow-up period was completed for the last patient in early 2017. The study’s main results were presented at the Society of Interventional Radiology’s Annual Scientific Meeting in Washington, D.C., on March 6, 2017, and were subsequently published in the New England Journal of Medicine on December 7, 2017. As of mid-2018, the study’s Steering Committee is continuing to analyze the data and write secondary manuscripts.
Main Study Results
P Clot Trial Procedure
Among patients with acute DVT, the addition of pharmacomechanical catheter directed thrombolysis (the clot-busting treatment) to blood-thinning medications (anticoagulants) did not result in a lower risk of PTS but did result in a higher risk of major bleeding, so it should not be used as routine first-line treatment for DVT. However, the clot-busting treatment improved recovery from DVT (reduced leg pain and swelling), and also reduced the severity of PTS – these benefits were mainly limited to patients with the largest blood clots.
How Was the Study Designed?
ATTRACT was a Phase III, multicenter, randomized, open-label, assessor-blind, controlled clinical trial (see full study protocol). The study is registered at www.clinicaltrials.gov (NCT 00790335) and was performed under IND 103462 from the U.S. Food and Drug Administration (for the off-label use of tissue plasminogen activator). Patients with symptomatic proximal DVT that involved the iliac, common femoral, and/or femoral vein (i.e. large blood clots of the leg or pelvis) were randomized (randomly chosen like the flip of a coin) to either receive or not receive a clot-busting treatment called Pharmacomechanical Catheter-Directed Thrombolysis (PCDT). All patients, whether or not they received PCDT, received standard treatment for their DVT, consisting of blood-thinning drugs and elastic compression stockings.
Patients were followed for 2 years, and had the following clinical outcomes rigorously assessed: the development and severity of PTS (Villalta scale, Venous Clinical Severity Score); change in health-related quality of life from baseline (VEINES-QOL/Sym and SF-36 measures); change in initial leg pain (Likert scale) and swelling (calf circumference) from baseline; safety (bleeding, recurrent DVT, death during follow-up), and costs. An ultrasound substudy was also performed in approximately one-sixth of the overall cohort to evaluate whether PCDT influenced rates of valve reflux and late venous obstruction, and whether this influenced PTS and quality of life.
Who Conducted the Study?
The ATTRACT Trial has been hailed as featuring an unprecedented degree of collaboration among international leaders in DVT research, education, and clinical practice from a broad range of clinical and non-clinical disciplines. The main collaborating institutions that ran the study were Washington University in St. Louis, MO (Clinical Coordinating Center), McMaster University in Hamilton, Ontario (Data Coordinating Center), Massachusetts General Hospital in Boston, MA (VasCore, Vascular Ultrasound Core Laboratory), and the University of Missouri – Kansas City, MO (Mid America Heart Institute, Health Economic Core Laboratory).
P Clot Trial Test
A full list of study contributors, including institutions, investigators, and research teams at the 56 Clinical Centers that enrolled patients in the study, can be found in this Online Supplement.
Study Leadership – Steering Committee
- David Cohen, MD, MSc (University of Missouri – Kansas City) – Co-Chair, Economic Core Lab
- Anthony J. Comerota, MD (University of Michigan) – Site Monitoring & Enrollment Committees
- Samuel Z. Goldhaber, MD (Harvard Medical School) – Chair, Steering Committee
- Heather Gornik, MD (Cleveland Clinic) – Chair, Medical Therapy Committee
- Michael R. Jaff, DO (Harvard Medical School) – Chair, Vascular Ultrasound Core Lab
- Jim Julian, MMath (McMaster University) – Lead Biostatistician
- Susan Kahn, MD, MSc (McGill University) – Chair, Clinical Outcomes Committee
- Clive Kearon, MB, PhD (McMaster University) – Chair, Data Coordinating Center
- Stephen Kee, MD (University of California, Los Angeles) – SIR Foundation Representative
- Andrei Kindzelski, MD, PhD (National Heart Lung and Blood Institute) – Project Officer
- Lawrence Lewis, MD (Washington University in St. Louis) – Chair, Enrollment Committee
- Elizabeth Magnuson, ScD (University of Missouri – Kansas City) – Economic Core Lab
- Timothy P. Murphy, MD (Brown University) – Operations and Site Monitoring Committees
- Mahmood K. Razavi, MD (St. Joseph’s Hospital) – Chair, Interventions Committee
- Suresh Vedantham, MD (Washington University in St. Louis) – National Principal Investigator
P Clot Trial
Who Funded and Supported the Study?
P Clot Trial Meaning
The ATTRACT Trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI), a part of the U.S. National Institutes of Health (NIH), via grants U01-HL088476 (to Dr. Suresh Vedantham from Washington University in St. Louis) and U01-HL088118 (to Dr. Clive Kearon at McMaster University).
The NHLBI provides global leadership for research, training, and education to promote prevention and treatment of heart, lung, and blood diseases and enhance the health of all individuals so that they can live longer and more fulfilling lives. The NIH, a part of the U.S. Department of Health and Human Services, is the primary Federal agency for conducting and supporting medical research. Helping to lead the way toward important medical discoveries that improve people’s health and save lives, NIH scientists investigate ways to prevent disease as well as the causes, treatments, and even cures for common and rare diseases.
Four companies provided supplemental support to the study: Boston Scientific Corporation (funding), Covidien (now Medtronic) (funding), Genentech (a Roche Company, donation of tissue plasminogen activator), and BSN Medical (donation of elastic compression stockings). The Society of Interventional Radiology (SIR) Foundation played a pivotal role in the genesis of ATTRACT and its Clinical Center network, and actively collaborated with the research team to educate physicians, the public, and the media about the trial, and to disseminate the study results.